39 labeled parts of a chemical equation
Chemical Equation | Reactants And Products In Chemical Reactions A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation. Law of conservation of mass governs the balancing of a chemical equation. Label the chemical Equation worksheet - Liveworksheets.com Live worksheets > English. Label the chemical Equation. Label the parts of the chemical Equation. ID: 1570824. Language: English. School subject: Science. Grade/level: 5. Age: 7-9. Main content: Chemical Equation.
Examples of Balanced Chemical Equations - ThoughtCo A balanced equation contains the same number of each type of atoms on both the left and right sides of the reaction arrow. To write a balanced equation, the reactants go on the left side of the arrow, while the products go on the right side of the arrow. Coefficients (number in front of a chemical formula) indicate moles of a compound.

Labeled parts of a chemical equation
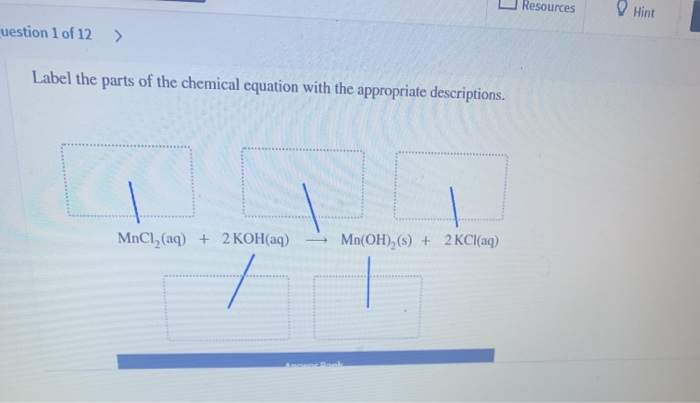
What is a Chemical Equation? - Definition & Examples To represent a generic chemical equation, we often use alphabetic letters. We read the equation as ''A plus B yields AB'' (or ''A plus B forms AB'' or ''A plus B goes to AB''). Note that A and B... Chemical Nomenclature and Chemical Formulas - Owlcation A chemical formula clearly shows the composition of a substance. It not only reveals the identity of the constituent elements, but also their fixed ratio by mass. The chemical formula also stands for one molecule of a substance. In a chemical equation, the formula represents one mole of a reactant or product. Solved label the parts of the chemical equation with the | Chegg.com Expert Answer 100% (2 ratings) Transcribed image text: On 7 of 29 Label the parts of the chemical equation with the appropriate descriptions. MnCl, (aq) + 2 KOH (aq) Mn (OH), (s) + 2 KCl (aq) Answer Bank aqueous solution product solid reactant liquid chemical change Previous question Next question
Labeled parts of a chemical equation. How to Write a Chemical Equation (with Pictures) - wikiHow The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2 Switch the ions to build the products. How to Read Chemical Formulas & Equations - Barista Hustle Ca + Cl 2 → CaCl 2 Calcium + chlorine → Calcium chloride If there are two or more of any molecules, that's indicated with a number in front of the molecule: CH 4 + 2O 2 → CO 2 + 2H 2 O One methane molecule + two oxygen molecules → one carbon dioxide molecule and two water molecules Phases Answered: Label the following parts of the… | bartleby Label the following parts of the chemical equation below: coefficient, subscript, products, reactants, yields, chemical formula. A. B. A. С. В. C. CaCl, + H,0 Cao + 2HCI D. E. D. E. F. F. The Law of Conservation of Mass states that during a chemical reaction, mass/matter is not Because of this law, there must be exactly Jon each side of a chemical ... Chem - Types of Chemical Equations | Scientific Tutor The first kinds of questions you want to be able to answer with the above information is if you are given a complete chemical equation. You want to be able to identify which one of the 5 types of chemical equations it is. To do that the best way is to ask questions about the chemical equations in front of you.
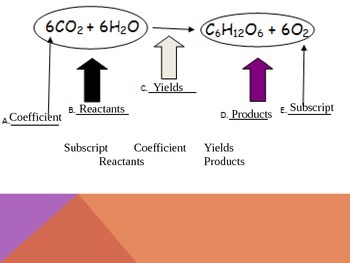
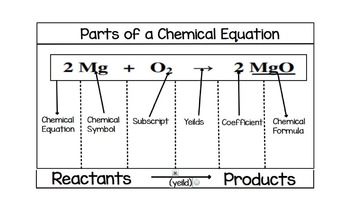
Parts of a chemical Equation- Write an equation and label the following ... Coefficient, subscript, Atoms, molecules, Reactants, and products are parts of a balanced chemical equation. Balanced chemical equation 6CO₂ ₊ 6H₂O=1C₆H₁₂O₆₊61O₂. Coefficient: A multiplier or factor that measures some property. Parts of a Chemical Reaction Flashcards | Quizlet Parts of a Chemical Reaction. STUDY. Flashcards. Learn. Write. Spell. Test. PLAY. Match. Gravity. Created by. azdanowicz. Terms in this set (6) Reactants. Substances at the beginning of a reaction. Products. Substances at the end of a reaction. Coefficients. Number in front of the formula that shows how many molecules in that formula ... Parts of Chemical Equation.docx - Course Hero Determine the limiting reactant and theoretical yield of MgO, when 3.07 g of Mg reacts with 4.09 of O2 in the following chemical equation: 2Mg + O2 ---> 2MgO Q&A An ice cube at 0.00 C with a mass of 4.52 is placed into 55 g of water, initially at 23 C. 3 Steps for Balancing Chemical Equations - ThoughtCo 1) Write the unbalanced equation. SnO 2 + H 2 → Sn + H 2 O Refer to Table of Common Polyatomic Ions and Formulas of Ionic Compounds if you have trouble writing the chemical formulas of the products and reactants. 2) Balance the equation. Look at the equation and see which elements are not balanced.
Solved Label the parts of the chemical equation with the | Chegg.com Question: Label the parts of the chemical equation with the appropriate descriptions. KOH (aq) + HI (aq) + H, O (1) KI (aq) Answer Bank liquid solid aqueous solution gas reactant chemical change product This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (3 ratings) What are the Parts of a Chemical Equation? - Life Persona Basically there are three Main parts in a chemical equation : The reactants, the products and the arrow indicating the direction of the chemical reaction. A chemical equation is an abbreviated form of representing the components of a chemical reaction. 4.1 Writing and Balancing Chemical Equations - Chemistry The numbers of H atoms on the reactant and product sides of the equation are equal, but the numbers of O atoms are not. To achieve balance, the coefficients of the equation may be changed as needed. Keep in mind, of course, that the formula subscripts define, in part, the identity of the substance, and so these cannot be changed without altering the qualitative meaning of the equation. What are Chemical Equations? Detailed Explanation, Examples Chemical Equation: CaCl 2 + 2AgNO 3 → Ca(NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3 - → Ca 2+ + 2NO 3 - + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ (calcium ion) and the NO 3 - (nitrate) ions are present on both sides of the ionic equation. These ions are referred to as spectator ions because they do not participate in the chemical reaction.
What are parts of a chemical reaction? - Answers Label all the parts of a chemical reaction? ... A chemical equation is defined as the short-hand representation of a true chemical reaction with the help of symbols and formula.A chemical equation ...
Parts of a Chemical Equation | Chemistry Quiz - Quizizz answer choices. The end result of a chemical reaction. The arrow that separates the product from the reactant. The number to the lower right of an element that shows the number of atoms. The substance changed in a chemical reaction. Tags: Question 4. SURVEY.
Chemical equation - Wikipedia A chemical equation (see an example below) consists of a list of reactants (the starting substances) on the left-hand side, an arrow symbol, and a list of products (substances formed in the chemical reaction) on the right-hand side.
Labeling A Chemical Equation Part 2 - YouTube Labeling A Chemical Equation Part 2 - YouTube.
What are the two parts of chemical formula? - Answers Ca2S is a chemical formula consisting of one part Calcium and two parts Sulfur. This chemical formula has a molar mass of 112.2210 g/mol. The Calcium mass percent of this formula is 71.4269%, while...
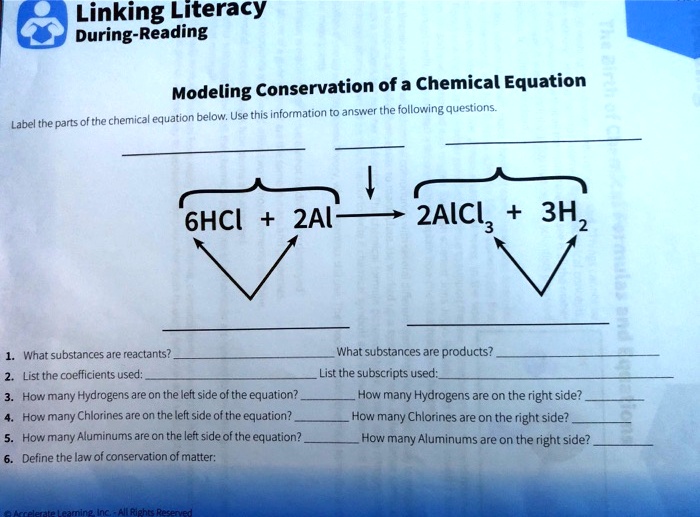
Linking Literacy During-Reading Modeling Conservation of a Chemical ... Linking Literacy During-Reading Modeling Conservation of a Chemical Equation Label the parts of the chemical equation below. Use this information to answer the following questions. - 6HCI + 2A 2AlCl, + 3H, 2 1. What substances are reactants? 2. List the coefficients used: 3. How many Hydrogens are on the left side of the equation? 4.
Parts Of A Chemical Equation Teaching Resources | TpT Parts of a Chemical Equation. by. Creativity Meets Cognition. 4.9. (101) $1.00. PDF. This fun Sketch Notes is an excellent addition to your interactive science notebook! The fun layout allows students to organize important information about the parts of a chemical equation while incorporating creativity into the classroom.
PLZ HELP ME FAST!!!! Identifying Parts of a Chemical Equation Label A leads to the beginning of the green box. Label B leads to the + in the blue box. Label C leads to the arrow. Label D leads to the 2 in 2 H subscript 2 O. Label E leads to the end of the pink box. Identify each part of this chemical equation, which describes the burning of methane and oxygen. A (the entire green box): B (the blue box):
Parts of a Chemical Equation Worksheet | Aurumscience.com. Parts of a Chemical Equation All of the basic parts of a chemical reaction are covered by this worksheet. Students will identify the reactants, products, subscripts, and coefficients. Included is information on the state of matter notation that indicates whether each substance is a solid, liquid, gas, or aqueous solution.
Solved label the parts of the chemical equation with the | Chegg.com Expert Answer 100% (2 ratings) Transcribed image text: On 7 of 29 Label the parts of the chemical equation with the appropriate descriptions. MnCl, (aq) + 2 KOH (aq) Mn (OH), (s) + 2 KCl (aq) Answer Bank aqueous solution product solid reactant liquid chemical change Previous question Next question
Chemical Nomenclature and Chemical Formulas - Owlcation A chemical formula clearly shows the composition of a substance. It not only reveals the identity of the constituent elements, but also their fixed ratio by mass. The chemical formula also stands for one molecule of a substance. In a chemical equation, the formula represents one mole of a reactant or product.
What is a Chemical Equation? - Definition & Examples To represent a generic chemical equation, we often use alphabetic letters. We read the equation as ''A plus B yields AB'' (or ''A plus B forms AB'' or ''A plus B goes to AB''). Note that A and B...
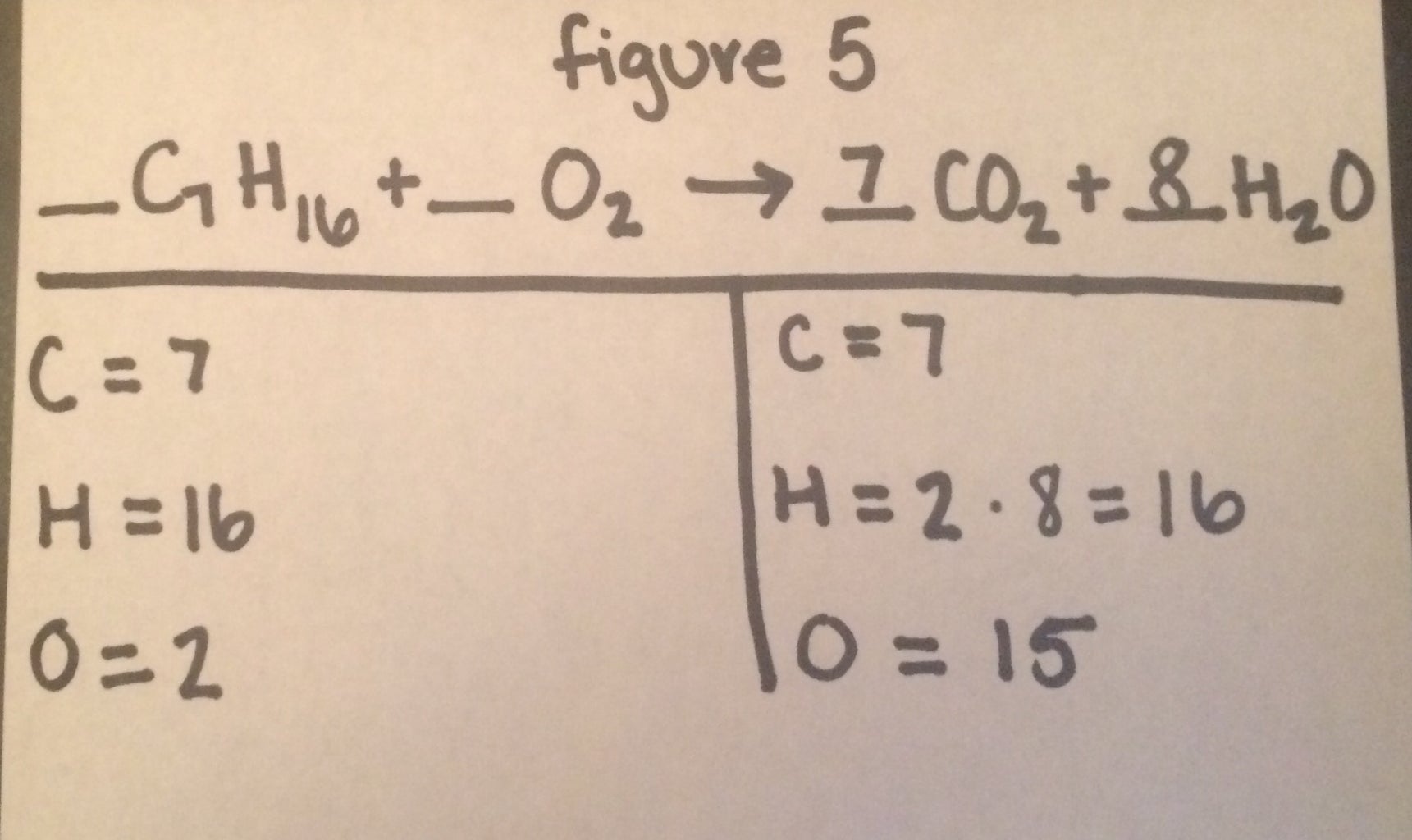
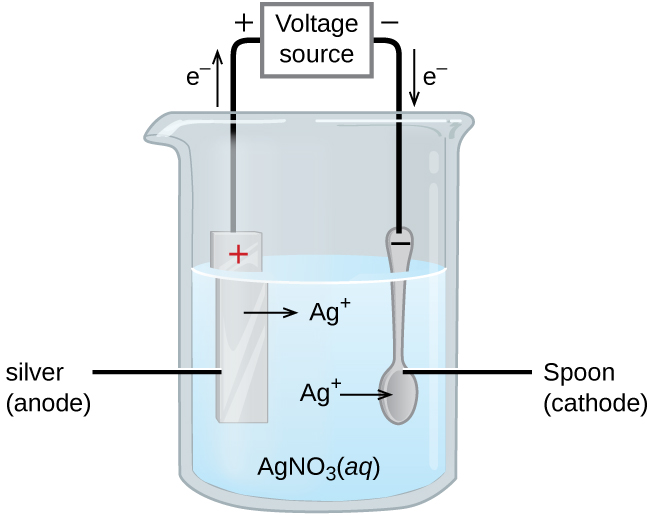


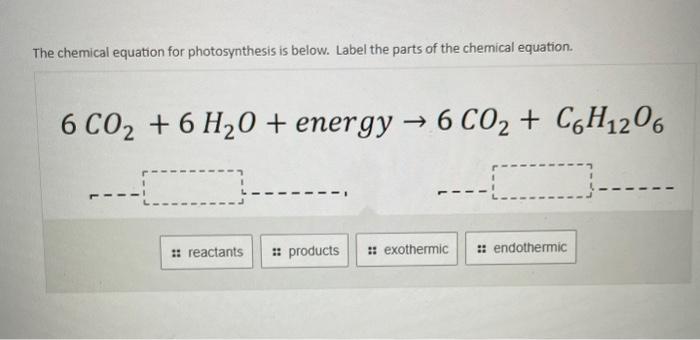
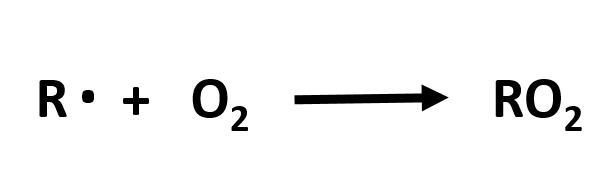




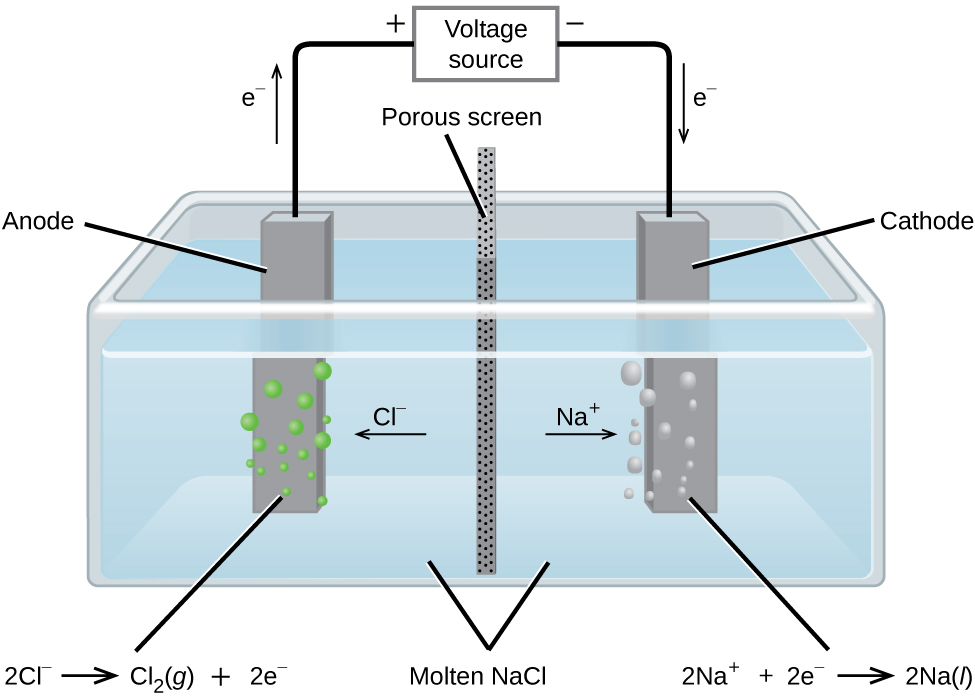

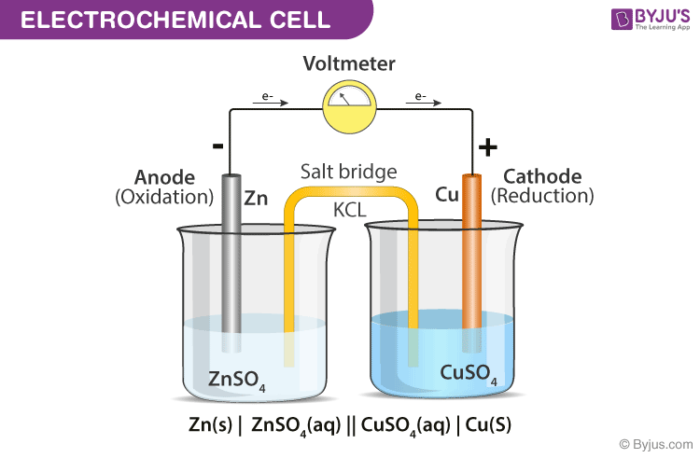





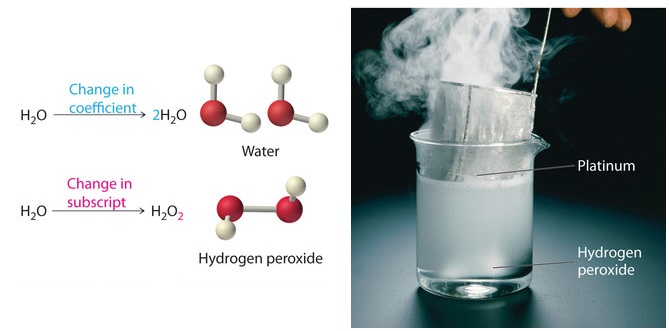
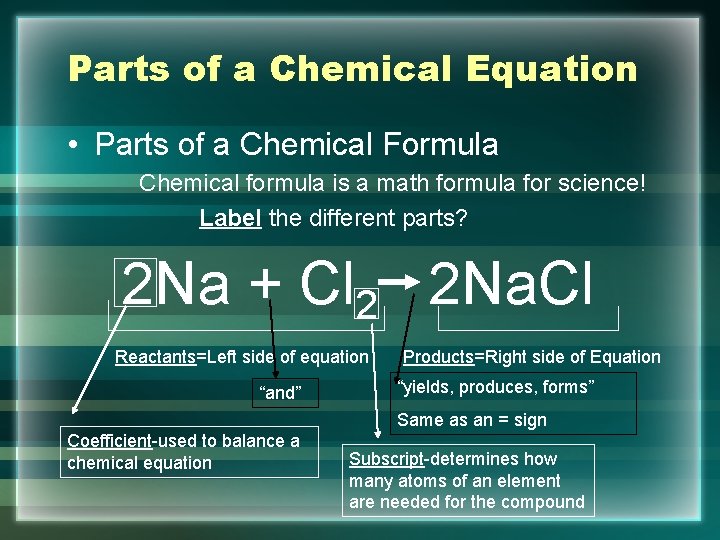






Post a Comment for "39 labeled parts of a chemical equation"