42 open-label study advantages and disadvantages
Reducing bias in open-label trials where blinded outcome assessment is ... Background Blinded outcome assessment is recommended in open-label trials to reduce bias, however it is not always feasible. It is therefore important to find other means of reducing bias in these scenarios. Methods We describe two randomised trials where blinded outcome assessment was not possible, and discuss the strategies used to reduce the possibility of bias. Results TRIGGER was an open ... National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.

Open-label study advantages and disadvantages
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely. Open-Label Extension Studies | SpringerLink The reputation of open-label extension study has been damaged by studies for which this has been the motivation. They have commonly been distinguished by rudimentary data collection and quality, revealing the true motive, namely market expansion. What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to continue ...
Open-label study advantages and disadvantages. External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random- Advantages and Disadvantages with Labelling Machines - AIS Ltd The device then shrinks back and the label is attached to your product. The advantage of using these types of labelling machines is that precision is excellent. This method is great when labelling difficult packaging products. The disadvantage is that this method of labelling can be slow and the labelling quality not as good as other methods. PDF Labeling and Disadvantages of Labeling - University of North Carolina ... scientists would be in raising money for cancer research if they had no name for it. The advantages of labeling can be summarized as follows: 1. Federal and local funding of special education programs are based on categories of disabilities. 2. Labeling enables professionals to communicate with one another because each categorical label Open-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
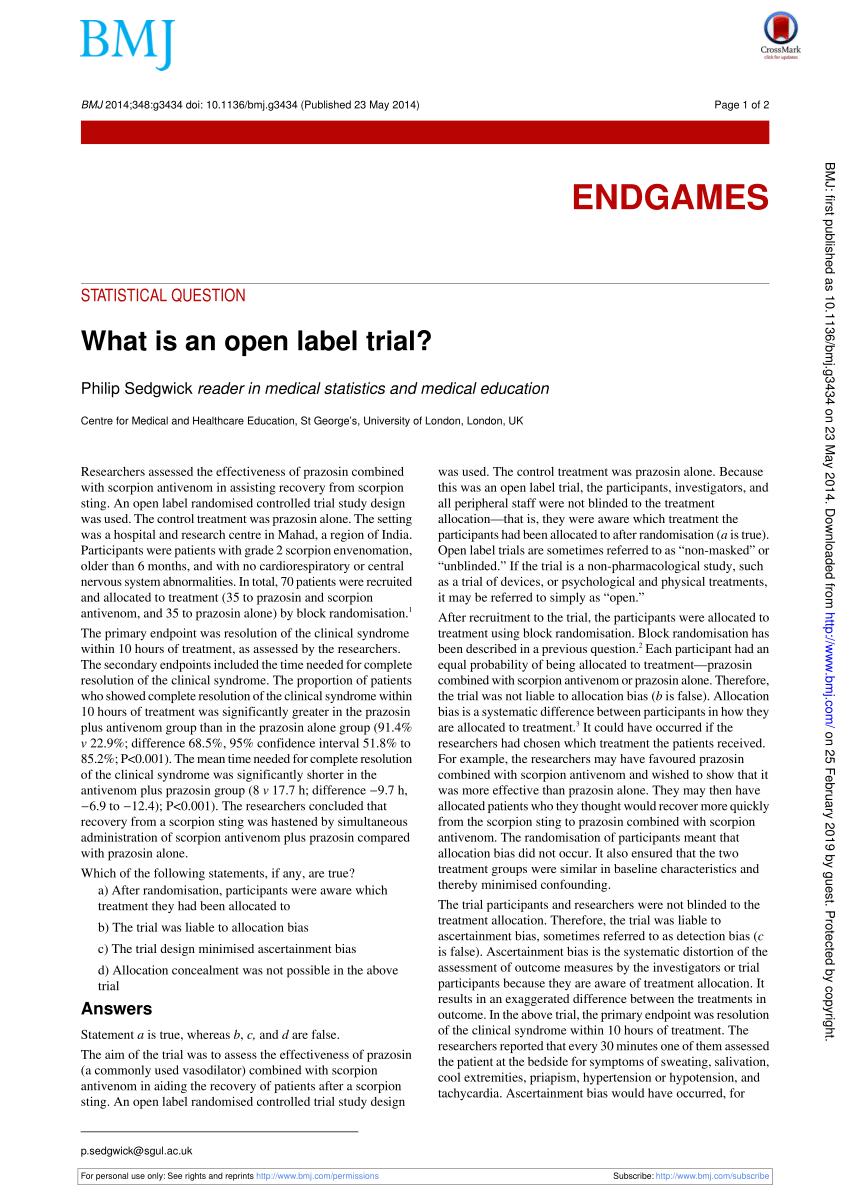
Open-Label Trial - an overview | ScienceDirect Topics An open-label trial has no blinding: everyone knows which patient is receiving which treatment. Open-label studies lack the rigor of blinded studies. External and internal validity of open label or double-blind ... - PubMed In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible. Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation.
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in addition to treatment assignment data itself, many other data elements in the CRF data can reveal treatment assignment. Those data elements include (but not limited to): • Self-Manage Scleroderma | Lesson Patients have a chance to help others and improve patient care. Some disadvantages might be: New treatments or interventions under study are not always better than, or even as good as, standard care. Even if a new treatment has benefits, it may not work for everyone. Health insurance and managed care providers don't always cover clinical trials. External and internal validity of open label or double ... - ResearchGate a blinded trial may be less subject to bias than an open-label trial because it minimizes the impact of knowledge of treatment allocation on postrandomized treatment decisions and on reporting of... product trial advantages and disadvantages - RHLC OGA Advantages Disadvantages Examples Umbrella Trial: De nition I Umbrella Trial: A master protocol where all patients (and all sub-trials) share a common tumor type (\the umbrella") 1 1Woodcock and LaVange. Open label study advantages and disadvantages. Advantages and disadvantages of single sex schools essay for addiction to the internet essay.
14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Disadvantages of Randomized Controlled Trials 1. The logistics of a randomized controlled trial can be demanding. Researchers and participants may need to endure a long trial run to ensure that there is enough data for comparison.
Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...
Study in China: advantages and disadvantages (Education in China) Education in China: advantages and disadvantages. When a person plans to study abroad, popular European universities immediately come to mind. At the same time, Chinese universities are in the TOP 3 countries, whose graduates find it easier to get a well-paid job. But studying in China also has other benefits:
The Advantages & Disadvantages of Unblinded Sample Size Re ... - Statsols The advantages of unblinded sample size re-estimation clinical trials. Earlier Decisions. You inherit the Group Sequential Design which allows the researcher to stop early for efficacy or futility. This allows the ability to adjust the trial to the reality of what the effect size is telling you will happen in the trial. Reduced Potential Cost.
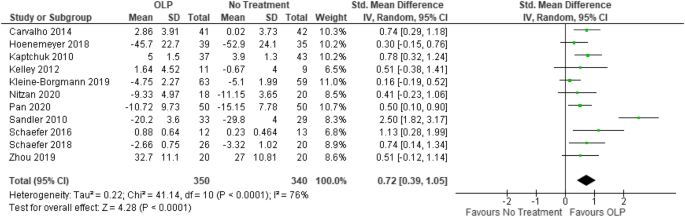
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to...
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: •
Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub-
External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias.
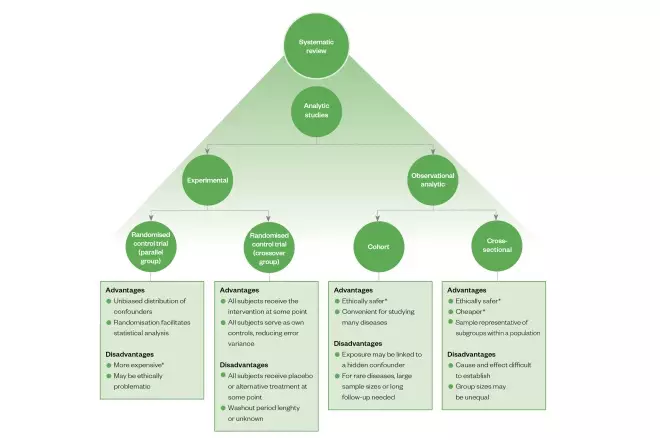
Study designs — Centre for Evidence-Based ... - University of Oxford An experimental comparison study in which participants are allocated to treatment/intervention or control/placebo groups using a random mechanism (see randomisation). Best for study the effect of an intervention. Advantages: unbiased distribution of confounders; blinding more likely; randomisation facilitates statistical analysis.
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to continue ...
Open-Label Extension Studies | SpringerLink The reputation of open-label extension study has been damaged by studies for which this has been the motivation. They have commonly been distinguished by rudimentary data collection and quality, revealing the true motive, namely market expansion.
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely.
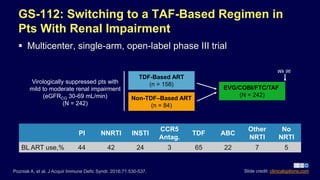





















![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)


Post a Comment for "42 open-label study advantages and disadvantages"