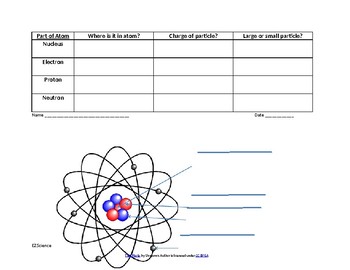
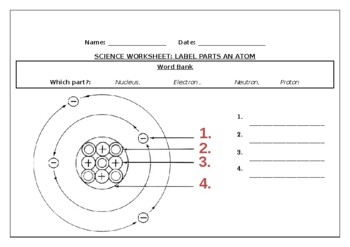
40 label the parts of atom
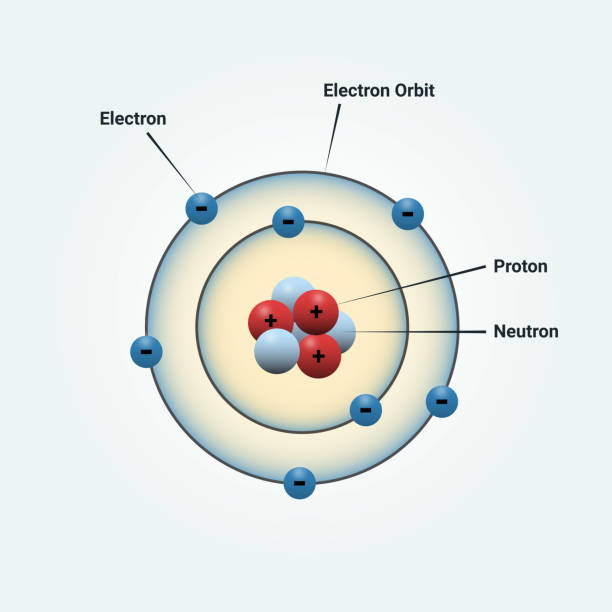
The Structure of an Atom: Parts, Diagram, Examples - Embibe Exams Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral). The electrons are negatively charged particles that orbit the nucleus's centre. Democritus came up with the concept that matter is composed of atoms. Atomic Structure - Electrons, Protons, Neutrons and Atomic Models - BYJU'S Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus.
What Are The Parts Of An Atom? - Universe Today Structure Of The Atom: Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with...

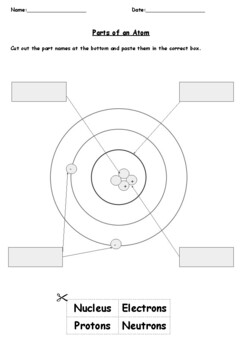
Label the parts of atom
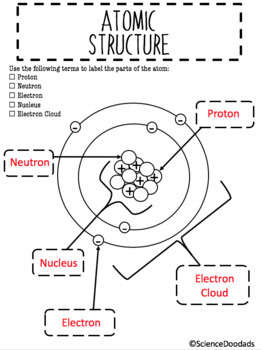
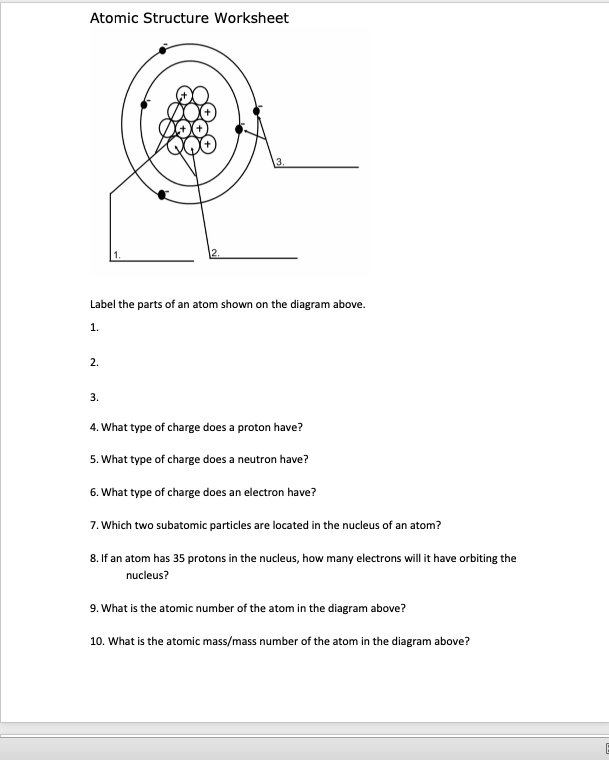
The periodic table, electron shells, and orbitals - Khan Academy By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid ... Atom: Definition, Structure & Parts with Labeled Diagram - Science Facts All atoms except hydrogen contain three basic subatomic particles: 1) electrons, 2) protons, and neutrons. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. 1) Electrons Label the Atom Diagram | Quizlet Center of the atom. Contains the protons and neutrons Electron Negative particles in the electron cloud. Discovered by JJ Thomson Neutron Particle with no charge in the nucleus of an atom. Discovered by Chadwick Electron Cloud Area outside the nucleus where electrons are located Proton Positive particles found in the nucleus of an atom.
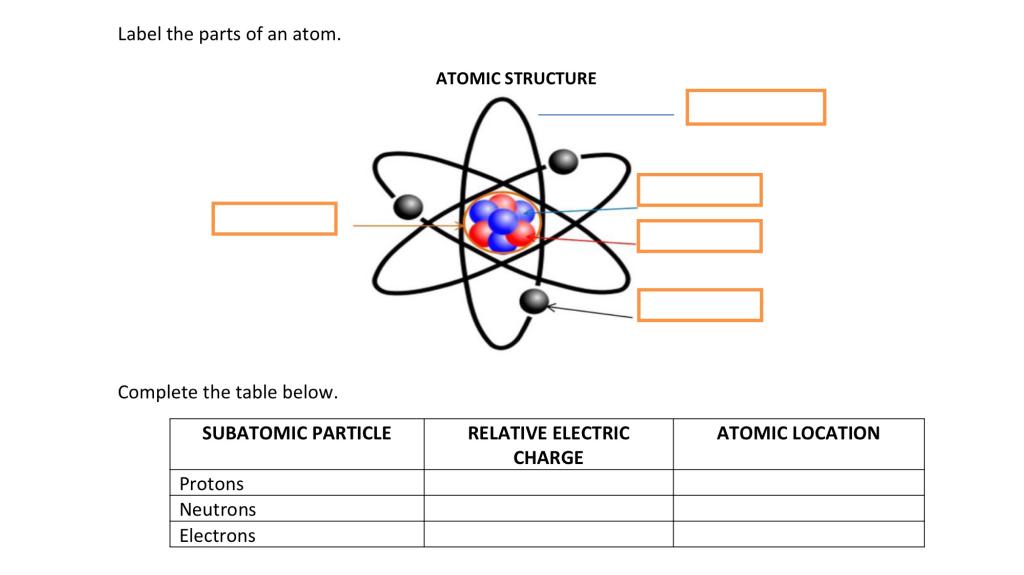
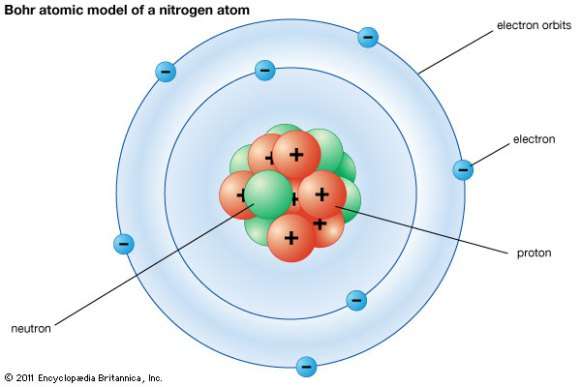
Label the parts of atom. Sub-Atomic Particles - Chemistry LibreTexts Sub-Atomic Particles. A typical atom consists of three subatomic particles: protons, neutrons, and electrons (as seen in the helium atom below). Other particles exist as well, such as alpha and beta particles (which are discussed below). The Bohr model shows the three basic subatomic particles in a simple manner. Label An Atom Teaching Resources | TPT - TeachersPayTeachers The Crafty Science Teacher. 4.9. (22) $1.50. PDF. This worksheet shows a diagram of an atom. Students will use the word bank and descriptions to help them label the parts of the atom.This diagram covers the readiness science TEKS 8.5 A where the student is expected to describe the structure of the atom. Label The Parts Of An Atom Teaching Resources | TPT - TeachersPayTeachers The Parts of an Atom worksheet contains 3 pages of drawings, charts, and questions to help students understand the atom. Students define an atom, label the parts, describe and compare the protons, neutrons, and electrons, and determine the meaning of atomic number and mass number. This clear and concise lesson was effective in getting the ... Label the parts of an atom Diagram | Quizlet Label the parts of an atom 3.0 (4 reviews) + − Flashcards Learn Test Match Created by Jenny_Linde Teacher Terms in this set (5) Term electron Definition negatively charged particle of an atom found outside the nucleus Location Term neutron Definition Particle in an atom with no charge. Found in the nucleus. Location Term proton Definition
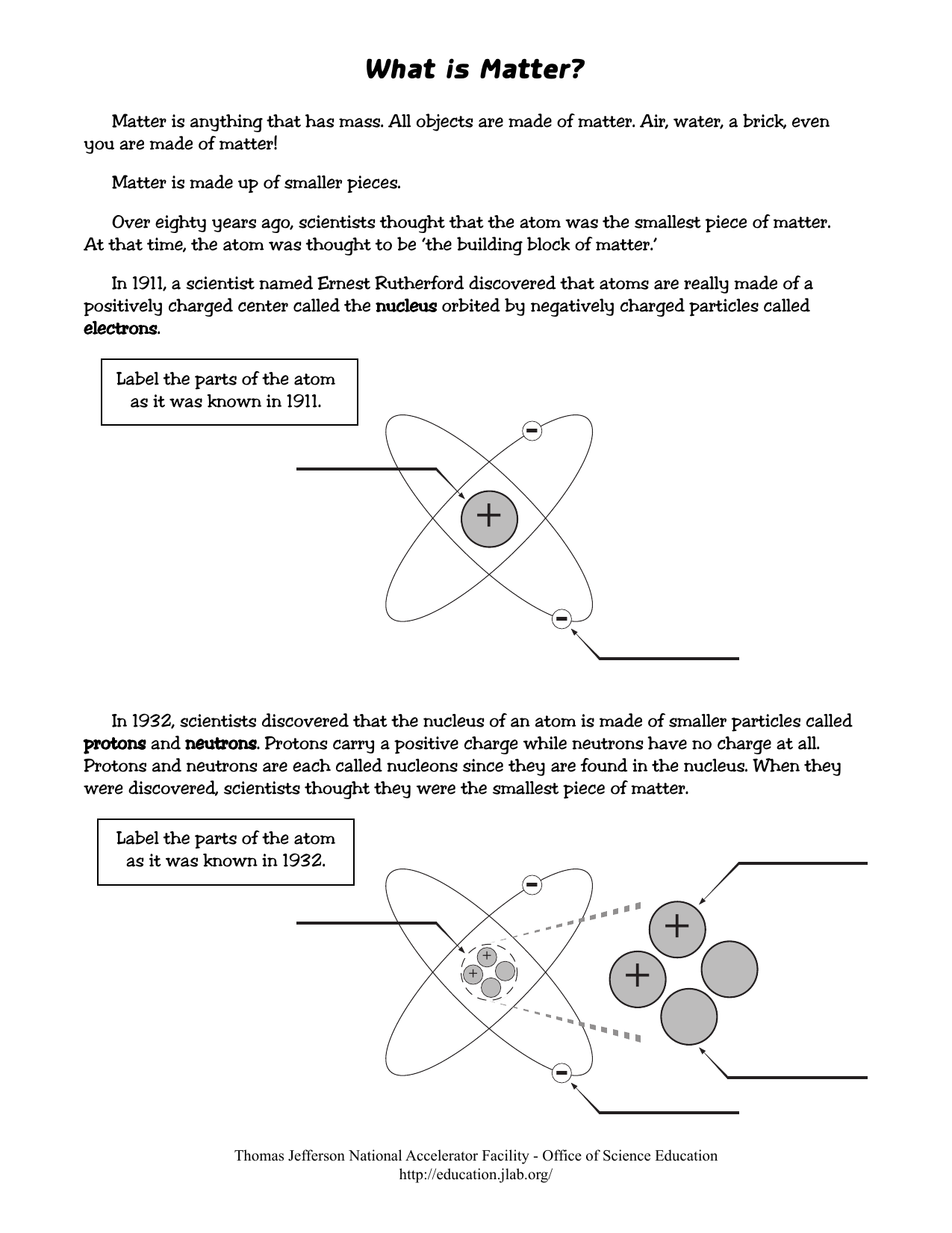
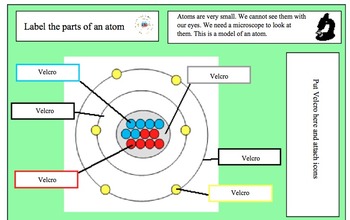
Basic Model of the Atom - Atomic Theory - ThoughtCo Atoms consist of three parts: Protons: Protons are the basis of atoms. While an atom can gain or lose neutrons and electrons, its identity is tied to the number of protons. The symbol for proton number is the capital letter Z. Neutrons: The number of neutrons in an atom is indicated by the letter N. Label Parts of an Atom — Learning in Hand with Tony Vincent Matter is composed of atoms, and atoms have three basic building blocks: protons, neutrons, and electrons. Protons have a positive charge, and neutrons have no charge. Protons and neutrons are in the nucleus of the atom. Electrons whiz around the center of the atom forming the electron cloud. Electrons have a negative charge. LabelthePartsandCharacteristicsofanatomandelement-1.doc - Label the ... Label the parts of an atom/element Created by: Patricia Hendrickson-When given a visual model of an atom, students will receptively identify protons, neutrons, electrons, nucleus and orbitals with 80% accuracy for 4 out of 5 trials. o When given a visual model of an atom, students will match protons, neutrons, electrons, nucleus and orbitals with color-coded prompts with 80% accuracy for 4 out ... Label the parts of an atom Diagram | Quizlet Label the parts of an atom + − Flashcards Learn Test Match Created by MaryBray5th Terms in this set (5) electron negatively charged particle of an atom found outside the nucleus neutron Particle in an atom with no charge. Found in the nucleus. proton Positively charged particle found in the nucleus of an atom nucleus
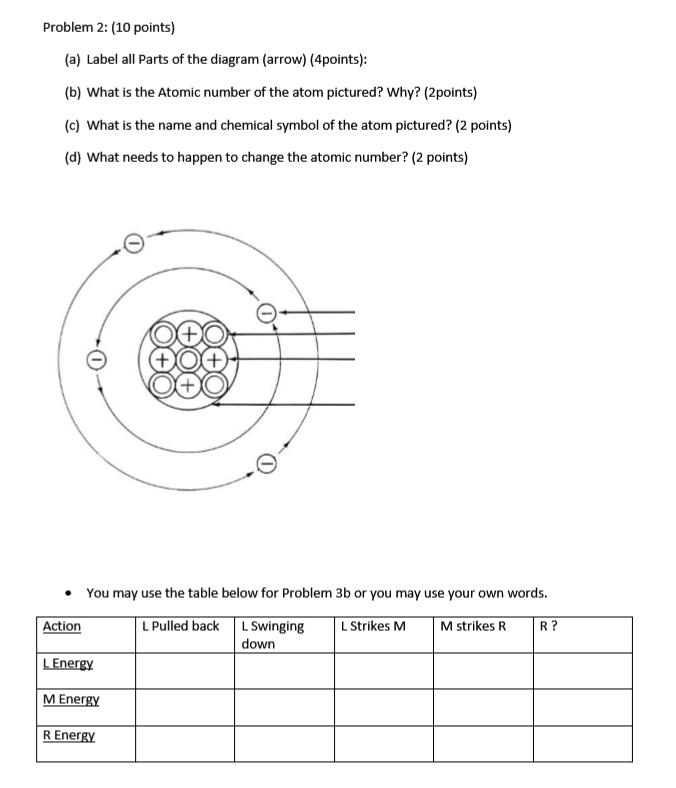
Sixth Grade Label Parts of an Atom Worksheet | Twinkl USA Atoms are mostly empty space, with the nucleus making up almost the entire mass of an atom. There are more than 100 types of atoms, with 92 of them being naturally occurring and the rest are man-made. The first man-made atom was technetium made in 1937. The above video is from a third-party source. 3.3: Subatomic Particles - Electrons, Protons, and Neutrons Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Masses for the three subatomic particles can be expressed in amu ( atomic mass units) or grams. For simplicity, we will use the amu unit for the three subatomics. The Structure of an Atom Explained With a Labeled Diagram With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. They could explain that an atom is made up of electrons, neutrons, and protons. The center of an atom is the nucleus that contain protons and neutrons. This makes the nucleus positively charged. Atomic number, atomic mass, and isotopes - Khan Academy Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom's mass number is its atomic mass.
3.4: Atomic Mass and Atomic Number - Chemistry LibreTexts Atomic Number. The atomic number (represented by the letter Z) of an element is the number of protons in the nucleus of each atom of that element.An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a ...
What is a Label Atom? The 4 Best Features - label template A label atom is a specific type of atom in the periodic table with a special chemical symbol and a unique atomic number. Unlike other atoms with a single nucleus, atom labels have two nuclei. These nuclei are called the label parts, each with a different symbol.
Atom | Definition, Structure, History, Examples, Diagram, & Facts Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together.
Label the Atom Diagram | Quizlet Center of the atom. Contains the protons and neutrons Electron Negative particles in the electron cloud. Discovered by JJ Thomson Neutron Particle with no charge in the nucleus of an atom. Discovered by Chadwick Electron Cloud Area outside the nucleus where electrons are located Proton Positive particles found in the nucleus of an atom.
Atom: Definition, Structure & Parts with Labeled Diagram - Science Facts All atoms except hydrogen contain three basic subatomic particles: 1) electrons, 2) protons, and neutrons. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. 1) Electrons
The periodic table, electron shells, and orbitals - Khan Academy By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid ...
.png_img_upload_solution_2022-06-26%2007:07:29.934790.png)




















Post a Comment for "40 label the parts of atom"